
Expanded perlite filter aid plays an essential role in the gold and silver mining process.
Among the many valuable applications of expanded perlite, mineral processing, specifically for gold and silver extraction, is among the most important. In the most common extraction method, gold and silver are leached from the ore using cyanide solution then precipitated with zinc powder or adsorbed on activated carbon. In either process, solid-liquids separation via filtration provides an integral step to carbon recovery, precipitates collection, or pregnant solution clarification.
Filter aids made with perlite offer a critical performance boost to any filtration process. Filter aids are used either as precoat to form a permeable layer on the filter septum before filtration initiation, or as body feeds (or body aids) during the filtration process. They are vital additives to the process because their use enhances filtration performance in a variety of ways.
Filter aids improve the permeability of the filter cake, give the filtrate more clarity, and help prevent the septum from blinding. Perlite filter aid is widely used in such applications, applied either as precoat on the septum and/or as body feed in the liquid, providing enhanced performance due to its unique properties.

The Merrill-Crowe Process
The Merrill-Crowe process, also called the zinc dust cementation process, is a widely used method for its simple and efficient operation for gold and silver recovery from cyanide leachate solution. The unit operations of the Merrill-Crowe process include: solid-liquid separation, clarification, vacuum de-aeration, zinc addition and filtration of precipitated gold and silver. The entire process is depicted in Figure 1.
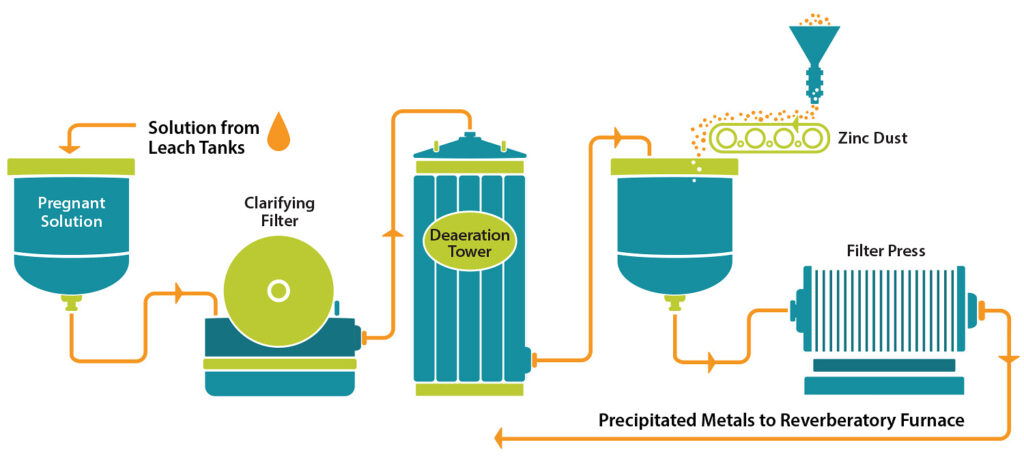
After the ore is leached with cyanide, the pregnant solution is filtered to remove solids in suspension. Solid-liquid separation is applicable only when heap leaching is applied and is carried out using thickeners. The clarified solution is then de-oxygenated in a vacuum tower known as a Merril-Crowe Tower. Zinc dust is then added to the solution of gold cyanide using a belt conveyor or vibrating tables. A reaction ensues wherein gold is precipitated out from the cyanide solution and deposited on the surface of the zinc—the cementation process. The zinc-gold particles are then large enough to be mechanically separated using a filter press.[1]
As noted above, filtration takes place in several stages, both when clarifying the pregnant solution, and later when separating the precipitated metals. The pregnant solution is first clarified in filters such as horizontal leaf type clarifiers. Precoating allows the production of an extremely clear solution. For efficiency, it is recommended to use two filters; one which is in use, and another which is being cleaned, precoated, and readied for service as soon as the first is brought offline. The solids removed by these filters are of no value and are backflushed to tails.
Final filtration of the precious metals is accomplished by the precipitate filter feed pumps and filter presses. Most filters are either the plate and frame type or the recessed plate type. Filter cake is collected in the chambers between the plates and can be air blown to remove a substantial amount of water. In a continuous operation, a process will have several filter presses in parallel so that one filter can be cleaned while the others are kept in operation, typically on a five- to seven-day cycle.
Carbon-in-Pulp (CIP) and Carbon-in Leach (CIL) Methods
Gold also has a natural affinity for carbon, and because of this, another common industrial practice is gold recovery from cyanide solution by adsorption on activated carbon. Two main variants of the process are in use today: the Carbon-in-Pulp (CIP) method, and the Carbon-in-Leach (CIL) method.[2]
Carbon-in-Pulp (CIP) is the sequential leaching then absorption of gold from ore. During the CIP process, pulp flows through several agitating tanks where sodium cyanide and oxygen have been added to dissolve gold into a solution. In the absorption stage, this solution flows through several agitating tanks containing activated carbon. Gold absorbs onto the activated carbon, which flows countercurrent to the pulp, while screens separate the barren pulp from the gold-loaded carbon.
Carbon-in Leach (CIL) differs from CIP in that it is a simultaneous leach and absorption process. The CIL process was developed in response to gold ores that contain material that consumes too much of the pregnant solution, such as natural absorptive carbon. This type of carbon affects yield by attracting gold meant instead for the activated carbon. The simultaneous leaching and absorption process of CIL helps to minimize this problem.

Perlite’s Role in Filtration
Perlite filter aid enhances the performance of the above processes in the following ways:
- For the removal of carbon fines generated during the many adsorption process steps, thus keeping all of the internal screens and filters free from premature plugging with carbon fines.
- For the concentration of carbon solids and removal of additional spent cyanide solution, aiming at the efficient recovery and dewatering of the carbon before the regeneration step.
- For the recovery of other metals (Pt, Cu, Mn, Co, Ni, etc.), which follow the precipitation step and subsequent solid-liquid separation through filtration.
Perlite Filter Aid Explained
Perlite filter aid is created through the expansion of perlite ore, the milling of that expanded perlite, and the classification of milled expanded perlite using cyclonic air separation. A range of perlite filter aid grades are produced, in terms of size, with varying technical properties to optimize filtration capacity for a wide variety of applications. The various grades utilize the jagged interlocking structures of the particles to create billions of microscopic channels between the filter aid particles (Figure 3). Perlite filter aids have gained acceptance in almost every industry concerned with the separation of liquids and solids, and even gasses and solids. In the mineral processing sector, perlite and diatomaceous earths are the most widely used filter aids.

Perlite filter aids have the following advantages:
- Low filter cake density (< 270 kg/m³)
- Grades can be tailored to custom applications
- Wide permeability range
- Less cake cracking and easy cake release
- More economical (as much as half as much mass is needed per unit of volume)
- Sterile and inert
- Safe to handle and easy to dispose
Perlite vs. Diatomaceous Earth (DE)
Perlite filter aids are functionally like DE filter aids; in fact, both are used interchangeably when the collected solids are not wanted for subsequent treatment. However, the different origin and physicochemical properties render perlite advantageous in the following ways:
- Perlite has 30-50% less bulk density, resulting in 30-50% less mass needed for a given filtration application.[3], [4]
- A wide network of perlite suppliers assures quick and uninterrupted local supply.
- Perlite filter aid contains little to no crystalline silica, thus posing less of a health risk.
- Perlite filter cake is not considered hazardous, thus not subject to strict disposal regulations.[5]
- Perlite filter aid contains significantly lower soluble iron, which can be detrimental to some processes.[5]
More information on Perlite Filter Aids, their properties, and their applications, can be found on the Perlite Institute website at www.perlite.org in the following brochures:
- Perlite for Filtration
- Perlite Filter Aid (PFA) for Vacuum & Pressure Filtration
- Perlite Filter Aids: Explained
- Perlite Filter Aid and the Circular Economy

BIBLIOGRAPHY
[1] G. Chi, M. C. Fuerstenau, and P. A. Anderson, “Study of Merrill-Crowe processing. Part II: Regression analysis of plant operating data,” Int. J. Miner. Process., vol. 49, no. 3–4, pp. 185–192, 1997.[2] W. Stange, “The process design of gold leaching and carbon-in-pulp circuits,” J. South African Inst. Min. Metall., vol. 99, no. 1, pp. 13–25, 1999.
[3] N. P. Cheremisinoff, “Filter Media and Use of Filter Aids,” Liquid Filtration, pp. 19–58, 1998.
[4] B. A. Perlmutter, Solid-Liquid Filtration: Practical Guides in Chemical Engineering. 2015.
[5] R. S. Jackson, Post-Fermentation Treatments and Related Topics. 2014.
To download a .pdf of the Extractive Metallurgy brochure, click here.
If you have technical questions on this topic, please email the technical contacts listed on our contact page.
Copyright © 2022 Perlite Institute All Rights Reserved
